Use this site to answer the questions about cephalopods!
All About Science
Tuesday, May 6, 2014
Mollusca- Bivalvia
Use this resource from UCMP to answer the questions about Bivalves related to diversity, environment, feeding, and more! Use this site for information about their life cycle.
Bivalves are the double shelled animals from the phylum Mollusca! They are the second most diverse group of Molluscs (the gastropods being the first), and the third most diverse class of animals (remember, insects are first!). These animals play a HUGE role in water and coral reef health.
As an idea of their diversity, think about the movie Pirates of the Caribbean Remember the crew of the Black Pearl? They were all covered in a wide variety of bivalves:
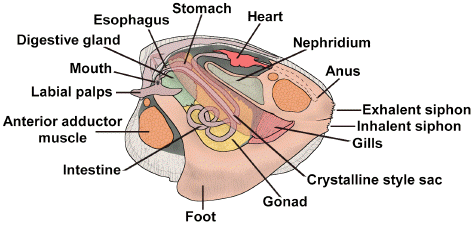
Here is a general body plan for the bivalves:
Questions:
Bivalves are the double shelled animals from the phylum Mollusca! They are the second most diverse group of Molluscs (the gastropods being the first), and the third most diverse class of animals (remember, insects are first!). These animals play a HUGE role in water and coral reef health.
As an idea of their diversity, think about the movie Pirates of the Caribbean Remember the crew of the Black Pearl? They were all covered in a wide variety of bivalves:
Here is a general body plan for the bivalves:
Questions:
- Give the Kingdom, phylum, and 6 important class names of bivalves.
- Where do bivalves live?
- Describe the body plan of the bivalves (these are in detail on the website).
- Remember, we said that the typical body plan "tool kit" of the molluscs includes 3 things. How does the bivalve body plan fit with that "tool kit"? - Does it have the same parts? What is different?
- Can bivalves move? If so, how?
- How do bivalves get nutrition and excrete waste?
- Do bivalves have sensory function? If so - how do they know what is going on around them?
- How do they reproduce? - What is their life cycle like?
- Why are bivalves so important?
Monday, April 21, 2014
Platyhelmenthes (the flat worms)
Platyhelminthes is a phylum of flatworms in the kingdom Animalia. While some species can be beautiful like the two directly below, more than half of the flatworm species are parasites infecting humans, pets, and livestock! Check out Animal Planet's Monsters Inside Me, on shape shifters here to learn about a few of the effects of flatworms in our daily lives.
Photo credit
Photo Credit
There are some features that all flatworms share.They have cephalization, including a distinct brain, and are bilaterally symmetrical. This means that we can tell the difference between anterior, posterior, dorsal, and ventral sides of flatworms. Platyhelmenths have no internal body cavity, meaning that they are acoelomates, but they do have 3 cell layers (endoderm, mesoderm, and ectoderm), just like humans. Below, you can see the body plan of a typical flat worm.
Photo Credit
Reproduction, feeding and sensory perception all very among the different classes of flatworms, but all classes rely on diffusion to get oxygen to their body cells.
4 classes of flatworms:
1. Turbellaria - like the planaria! There are around 4,500 species known in marine, fresh water and moist terrestrial habitats. Nearly all Turbellarids are hermaphrodites and reproduce sexually, though they can also reproduce asexually by fragmentation (similar to regeneration). Sexual reproduction in this class can be a little unusual, so much so that they made it onto "NatGeo Wild's" series World's Weirdest for a behavior called "penis fencing." If you're curious, here is the clip! These flatworms are the only class with specific sensory organs, and they have eyespots, which respond to light.
Photo Credit
2. Tremetoda -like flukes. There are as many as 24,000 known trematode species, and all are parasites which include 2 hosts in their lifecycle. Most species use snails as intermediate host before infecting fish and even humans. Depending on the species, flukes can infect the skin, blood, or organs like the lungs and liver. Freshwater is a prime habitat for infection. Occasionally, lakes are closed for swimming due to presence of the flukes in the water. In the USA, nearly all species will only infect the skin, causing a rash, because humans are not the primary host, but that is not the case when traveling to places where water is used for washing purposes. Check it out here from"Animal Planet's" Monsters Inside Me. Below is the lifecycle of a type of trematode knowns as schistosoma. The cycle is complicated but notice the key points:
A. Sexual Reproduction occurs in the human host
B. Eggs are excreted in human feces and urine and end up in water
C. Once in water, the eggs hatch and the young (miracidia) infect water snails.
D. The miracidia grow within the snail and enter the water in another form, the cercariae.
E. Humans in the water can become infected when the cercariae enter the skin.
F. Once in the human, the cercariae become adult worms, which mate and lay more eggs. --> the cycle continues
Photo Credit
3. Cestoda- the tapeworms! YUMMMM! This is another class of platyhelminths where all of the species are parasites. The tapeworm gains at the host's expense. An infected host will consume food, digest it, and then the tapeworm within the hose absorbs the nutrition from the food. Again, a class of Platyhelmenthes are so interesting that they made another prime time feature on "Animal Planet's" Monsters Inside Me. Check it out here!
Rumor has it that people once intentionally consumed tapeworms to lose weight because the worm would block in absorption of calories. Talk about an "organic" weight loss plan!? But look at this creature - would you really want that inside of you?! They have hooks on their head, which they bury into stomach or intestinal lining, and they grow... and they grow... and they grow... and the record is: almost 29 Feet! YUCK.
Photo Credit
Photo Credit
What is particularly scary is that there are a wide variety of ways to get infected, which depends on the species, and not all tape worms go for our guts. Some tapeworms live inside of fleas, which can jump off of your dogs and into your mouth, eventually infecting you. They can also enter the body through contaminated food (especially fish and pork). Certain species can even directly enter the skin (usually on cuts and sores). Depending on the species, the worms can live in the guts, skin, or even the brain. And, they can reproduce fast. Each body segment, called a proglottid has male and female parts, which can self fertilize, and when the proglottids break off, they can form all new worms! Ok, Nightmare? Here is the life cycle:
Photo Credit
4. Monogenea are a class of small flatworm which at ectoparasites (parasites that live on the outside) of marine fish fins and gills. Compared to the other three classes, they are very simple animals with no intermediate hosts, and only one species is know to infect an animal other than fish. That species actually infects the eye of hippopotamus!
Photo Credit
Photo credit
Photo Credit
There are some features that all flatworms share.They have cephalization, including a distinct brain, and are bilaterally symmetrical. This means that we can tell the difference between anterior, posterior, dorsal, and ventral sides of flatworms. Platyhelmenths have no internal body cavity, meaning that they are acoelomates, but they do have 3 cell layers (endoderm, mesoderm, and ectoderm), just like humans. Below, you can see the body plan of a typical flat worm.
Photo Credit
Reproduction, feeding and sensory perception all very among the different classes of flatworms, but all classes rely on diffusion to get oxygen to their body cells.
4 classes of flatworms:
1. Turbellaria - like the planaria! There are around 4,500 species known in marine, fresh water and moist terrestrial habitats. Nearly all Turbellarids are hermaphrodites and reproduce sexually, though they can also reproduce asexually by fragmentation (similar to regeneration). Sexual reproduction in this class can be a little unusual, so much so that they made it onto "NatGeo Wild's" series World's Weirdest for a behavior called "penis fencing." If you're curious, here is the clip! These flatworms are the only class with specific sensory organs, and they have eyespots, which respond to light.
2. Tremetoda -like flukes. There are as many as 24,000 known trematode species, and all are parasites which include 2 hosts in their lifecycle. Most species use snails as intermediate host before infecting fish and even humans. Depending on the species, flukes can infect the skin, blood, or organs like the lungs and liver. Freshwater is a prime habitat for infection. Occasionally, lakes are closed for swimming due to presence of the flukes in the water. In the USA, nearly all species will only infect the skin, causing a rash, because humans are not the primary host, but that is not the case when traveling to places where water is used for washing purposes. Check it out here from"Animal Planet's" Monsters Inside Me. Below is the lifecycle of a type of trematode knowns as schistosoma. The cycle is complicated but notice the key points:
A. Sexual Reproduction occurs in the human host
B. Eggs are excreted in human feces and urine and end up in water
C. Once in water, the eggs hatch and the young (miracidia) infect water snails.
D. The miracidia grow within the snail and enter the water in another form, the cercariae.
E. Humans in the water can become infected when the cercariae enter the skin.
F. Once in the human, the cercariae become adult worms, which mate and lay more eggs. --> the cycle continues
Photo Credit
3. Cestoda- the tapeworms! YUMMMM! This is another class of platyhelminths where all of the species are parasites. The tapeworm gains at the host's expense. An infected host will consume food, digest it, and then the tapeworm within the hose absorbs the nutrition from the food. Again, a class of Platyhelmenthes are so interesting that they made another prime time feature on "Animal Planet's" Monsters Inside Me. Check it out here!
Rumor has it that people once intentionally consumed tapeworms to lose weight because the worm would block in absorption of calories. Talk about an "organic" weight loss plan!? But look at this creature - would you really want that inside of you?! They have hooks on their head, which they bury into stomach or intestinal lining, and they grow... and they grow... and they grow... and the record is: almost 29 Feet! YUCK.
Photo Credit
Photo Credit
What is particularly scary is that there are a wide variety of ways to get infected, which depends on the species, and not all tape worms go for our guts. Some tapeworms live inside of fleas, which can jump off of your dogs and into your mouth, eventually infecting you. They can also enter the body through contaminated food (especially fish and pork). Certain species can even directly enter the skin (usually on cuts and sores). Depending on the species, the worms can live in the guts, skin, or even the brain. And, they can reproduce fast. Each body segment, called a proglottid has male and female parts, which can self fertilize, and when the proglottids break off, they can form all new worms! Ok, Nightmare? Here is the life cycle:
Photo Credit
4. Monogenea are a class of small flatworm which at ectoparasites (parasites that live on the outside) of marine fish fins and gills. Compared to the other three classes, they are very simple animals with no intermediate hosts, and only one species is know to infect an animal other than fish. That species actually infects the eye of hippopotamus!
Photo Credit
Sunday, April 13, 2014
Applications of Genetics - Making a Pegidree
This site, from Utah.edu is a WONDERFUL source for teaching and learning genetics. The site covers topics from general background information on genetics to human health, cell biology, evolution and ecology, and tools in science (complete with virtual labs and mathematical application!) .
Whats a pedigree? Practice creating and understanding pedigrees with this worksheet. (Open the .doc here)
This week, we are working with pedigrees in order to get a better understanding of inheritance and gene flow from one generation to the next. Students will complete their own pedigrees using the iPad app "Power Lineage." This app is GREAT because it is not only a fun tool for learning more about genetics, but it is also HIPAA Compliant so that personal information remains private. It can trace disease and heredity for many genes and provide individual analysis.
To begin, students will make their possible genotypes for the observable human characteristics described here. Then, students will begin to complete the genotypes for their family members, in order to great a more complete picture of their own alleles.When determining alleles, be sure to add in the "Widow's Peak," shown below.
Students will Also add their blood type information. Here is a great key to explaining the role of genetics in blood type.
How does a seemingly useless gene play a role in evolution of traits? Click here to find out more about the PTC test (and why tasting such a disgusting piece of paper could really be a good deal!).
Ask yourself now, does addiction run in your family? Read more about it here to find out how you can discover the answer!
And what about mental illness?! This page is very informative and complete with podcast clips for audible explanations.
For the scary stuff - genetic disorders. There are many lists provided on the Utah site of various disorders. The lists are organized by the type of inheritance and each disorder has a separate link for more information about it. Single-Gene disorders (cystic fibrosis, Huntington's Disease, sickle cell disease, etc) are all on one list, while Chromosomal Abnormality disorders (Down Syndrome, Klinefelter Syndrome, and Turner's Syndrome) and on another. The site also has information about newborn screening and how it can help keep babies healthy!
Take a look here for more information about the genetic risk for disease. This page gives a list of different risk-associated diseases (like high blood pressure, heart disease, diabetes, and more), which each link out to more information about that disease! Also, you can learn more about how the environment, diet and activity, and cholesterol all play some role in a persons' risk for obtaining those diseases.
Later, we will apply these concepts to variation of traits over time and evolution!
Whats a pedigree? Practice creating and understanding pedigrees with this worksheet. (Open the .doc here)
This week, we are working with pedigrees in order to get a better understanding of inheritance and gene flow from one generation to the next. Students will complete their own pedigrees using the iPad app "Power Lineage." This app is GREAT because it is not only a fun tool for learning more about genetics, but it is also HIPAA Compliant so that personal information remains private. It can trace disease and heredity for many genes and provide individual analysis.
To begin, students will make their possible genotypes for the observable human characteristics described here. Then, students will begin to complete the genotypes for their family members, in order to great a more complete picture of their own alleles.When determining alleles, be sure to add in the "Widow's Peak," shown below.
Students will Also add their blood type information. Here is a great key to explaining the role of genetics in blood type.
How does a seemingly useless gene play a role in evolution of traits? Click here to find out more about the PTC test (and why tasting such a disgusting piece of paper could really be a good deal!).
Ask yourself now, does addiction run in your family? Read more about it here to find out how you can discover the answer!
And what about mental illness?! This page is very informative and complete with podcast clips for audible explanations.
For the scary stuff - genetic disorders. There are many lists provided on the Utah site of various disorders. The lists are organized by the type of inheritance and each disorder has a separate link for more information about it. Single-Gene disorders (cystic fibrosis, Huntington's Disease, sickle cell disease, etc) are all on one list, while Chromosomal Abnormality disorders (Down Syndrome, Klinefelter Syndrome, and Turner's Syndrome) and on another. The site also has information about newborn screening and how it can help keep babies healthy!
Take a look here for more information about the genetic risk for disease. This page gives a list of different risk-associated diseases (like high blood pressure, heart disease, diabetes, and more), which each link out to more information about that disease! Also, you can learn more about how the environment, diet and activity, and cholesterol all play some role in a persons' risk for obtaining those diseases.
Later, we will apply these concepts to variation of traits over time and evolution!
Tuesday, April 8, 2014
Chemistry- Monatomic Ions Knowledge Quest Questions
Please excuse the funky margins and numbering. This was copied in from Word and I could not get it to translate correctly.
Name:
______________________ Date: ______________ Class Period: ______________
Knowledge Search Questions
Chapter
7 Chemical Formulas and Chemical Compounds
1.
How do atoms attain a full outer electron orbital?
a.
b.
c.
2.
What is a monatomic ion?
3.
What are the parts of an atom AND what charge does each part
have?
a.
b.
c.
4.
Give the overall charge for the following:
a.
2 electrons and 3 protons =
b.
3 electrons and 3 protons =
c.
1 electron and 3 protons =
d.
4 protons and 2 electrons =
e.
5 protons and 8 electrons =
5.
What is an ion?
6.
What is a cation?
7.
What is an anion?
8.
What is meant by the “noble-gas configuration?”
9.
Explain electron movement (gain or loss) in the following
element groups:
a.
Group 1
b.
Group 2
c.
Group 15
d.
Group 16
e.
Group 17
10.
Group 14 elements (C, Si, Ge, Sn, Pb) are not as straight
forward as the groups discussed in the question above. Explain the d-block atoms as ions.
11.
How are monatomic cations named? Give 3 examples.
12.
How are monatomic anions named? Give 3 examples
13.
In general, what causes atoms to be more or less
electronegative?
14.
When does an atom have low electronegativity?
15.
Explain the relationship between monatomic ion charge and
electron loss / gain.
16.
Explain the relationship between monatomic ion charge and
atom grouping on the periodic table.
17.
Explain how Hydrogen fits into monatomic ions?
18.
Why do some monatomic ions have roman numerals in their
names?
19.
Which group of elements could potentially contain roman numerals?
20.
When those elements discussed above form ions, are they
cations or anions?
21.
What is the d-block?
Chemistry- Naming Monatomic Ions Reading
Currently, this course using the following text:
Davis, R. E., & Holt, R. (1999). Modern chemistry: [Gr. 9-12]. Austin, Tex: Holt, Rinehart and Winston.
These photos were taken of the book from chapter 7, pages 204 and 205.
Page 204
Davis, R. E., & Holt, R. (1999). Modern chemistry: [Gr. 9-12]. Austin, Tex: Holt, Rinehart and Winston.
These photos were taken of the book from chapter 7, pages 204 and 205.
Page 204
Page 205
Chemistry- Naming Monatomic Ions
What is a monatomic ion?!
Watch the video here!
Let's get some background information.
Think back to electron configurations and bonding. Remember how all elements "strive" for balance - for that full outer orbital?
Many elements achieve this through chemical bonding, by either sharing electrons, stealing electrons, or giving up electrons to the partner in the bond.
There are some elements, however, that can give up electrons in their valance orbital to achieve the ideal full orbital! These elements can to this with one atom - thus they are called "monatomic ions." The prefix "mono" or "mon in this case" translates to 1 or single. Of course, atomic refers to a single atom. Thus, the term translates to single atom ions.
There are some results to this. But first, we need to take the time to really understand the parts of an electron, because a lot of naming and chemical reactions deals with charge. So, go here (an older blog of mine), read about charges. Do not worry so much about oxidation and reduction, we will get around to that, so you can stop reading when you read this part "Answer: the charge", and come back to this page.
So, to recap the important points, electrons are negative and protons are positive. Atoms with an unequal number of protons and electrons will have a charge; we call those ions. A positively charged ion is a cation, resulting from having fewer electrons than protons. Oppositely, an atom can gain electrons. Having a surplus of electrons compared to protons, then, results in an anion, which is a negatively charged ion. Take the equations below for example.
3 p - 3 e = 0 BUT 3 p - 2 e = 1 which is positive!
3 p - 3 e = 0 BUT 2 p + (-3 e) = -1 which is negative!
Read in your text book (Modern Chemistry by Holt, Rinehart and Winston, 1999) Chapter 7 pages 204 and 205 about Monatomic ions. If you do not have your text, click here for a link to photo image of the pages.
Based on the number of electrons in the valence shell, atoms will either give up or gain electrons. You have learned some about electronegativity in bonding, where elements at the left of the table are less electronegative and those at the right side are highly electronegative. This is because there are less outer electrons in left elements, and more in right elements. Look at the table below:
This table shows the elements that are able to engage in forming monatomic ions - those which are considered "main group" elements.
Note that above each column, the charge of the atom as an ION is provided. This means that the atom has gained or lost electrons - it has a charge.
Take a look at the forming of cations. What is the charge of a cation? (POSITIVE! less electrons)
To form cations, atoms from column 1 lose 1 electron, gaining a positive 1 charge. study the chart and write what happens for the other columns.
Now, let's look at anions. What is the charge of an anion? (NEGATIVE! more electrons)
Notice here that Hydrogen is included here. To obtain a full outer shell, Hydrogen will gain an electron. Hydrogen, though, is tricky. MOST of the time in chemical reactions, it will gain an electron, becoming an anion. However, the single electron that hydrogen carries is very often stripped away in biochemical reactions like photosynthesis so that the electron can be used as pure energy in the formation of sugar from the sun. As fascinating as that is, we will not go further into details about that, and for the sake of this course, consider H as forming anions.
NAMING!!!
So finally, now that you know which way these atoms swing in ion formation, we must name them.
Cations are simple. You just give the name of the element and add the word "cation" at the end.
Examples:
K+ is "Potassium cation" and Mg2+is "Magnesium cation"
Anions are a little different. Part of the end of the element's name is removed and an -ide ending is added. The word anion is NOT used.
Examples:
F- is "Fluoride" and N3- is "Nitride"
Below is a picture of a table from the course text (Davis, R. E., & Holt, R. (1999). Modern chemistry: [Gr. 9-12]. Austin, Tex: Holt, Rinehart and Winston.) on page 205. This table shows the common monatomic ions.
You will notice that in the d-block list of atoms, there are some names which include roman numerals, related to the stock system of naming elements and chemical ions. Here is a quick link to the rules (which we will discuss with section 7-2). In summary, the roman numerals are used when there are different possible charges for the cation. Meaning that sometimes, the atom might lose 2 elections (gaining a 2+ charge), while others it could lose 3 (gaining a plus 3 charge). In the first case, a numeral of II would represent the oxidation number of 2, while in the second case, there would be a numeral of III to represent that the ion for that particular element used in this case is the one which has lost 3 electrons.
When the table mentions "d-block," they are referring to the orbitals discussed in chapter 4. the d-block elements are transition metals, and are ofter very unique. Below is the periodic table with the orbitals provided to refresh your memory.
Watch the video here!
Let's get some background information.
Think back to electron configurations and bonding. Remember how all elements "strive" for balance - for that full outer orbital?
Many elements achieve this through chemical bonding, by either sharing electrons, stealing electrons, or giving up electrons to the partner in the bond.
There are some elements, however, that can give up electrons in their valance orbital to achieve the ideal full orbital! These elements can to this with one atom - thus they are called "monatomic ions." The prefix "mono" or "mon in this case" translates to 1 or single. Of course, atomic refers to a single atom. Thus, the term translates to single atom ions.
There are some results to this. But first, we need to take the time to really understand the parts of an electron, because a lot of naming and chemical reactions deals with charge. So, go here (an older blog of mine), read about charges. Do not worry so much about oxidation and reduction, we will get around to that, so you can stop reading when you read this part "Answer: the charge", and come back to this page.
So, to recap the important points, electrons are negative and protons are positive. Atoms with an unequal number of protons and electrons will have a charge; we call those ions. A positively charged ion is a cation, resulting from having fewer electrons than protons. Oppositely, an atom can gain electrons. Having a surplus of electrons compared to protons, then, results in an anion, which is a negatively charged ion. Take the equations below for example.
3 p - 3 e = 0 BUT 3 p - 2 e = 1 which is positive!
3 p - 3 e = 0 BUT 2 p + (-3 e) = -1 which is negative!
Read in your text book (Modern Chemistry by Holt, Rinehart and Winston, 1999) Chapter 7 pages 204 and 205 about Monatomic ions. If you do not have your text, click here for a link to photo image of the pages.
Based on the number of electrons in the valence shell, atoms will either give up or gain electrons. You have learned some about electronegativity in bonding, where elements at the left of the table are less electronegative and those at the right side are highly electronegative. This is because there are less outer electrons in left elements, and more in right elements. Look at the table below:
This table shows the elements that are able to engage in forming monatomic ions - those which are considered "main group" elements.
Note that above each column, the charge of the atom as an ION is provided. This means that the atom has gained or lost electrons - it has a charge.
Take a look at the forming of cations. What is the charge of a cation? (POSITIVE! less electrons)
To form cations, atoms from column 1 lose 1 electron, gaining a positive 1 charge. study the chart and write what happens for the other columns.
Now, let's look at anions. What is the charge of an anion? (NEGATIVE! more electrons)
Notice here that Hydrogen is included here. To obtain a full outer shell, Hydrogen will gain an electron. Hydrogen, though, is tricky. MOST of the time in chemical reactions, it will gain an electron, becoming an anion. However, the single electron that hydrogen carries is very often stripped away in biochemical reactions like photosynthesis so that the electron can be used as pure energy in the formation of sugar from the sun. As fascinating as that is, we will not go further into details about that, and for the sake of this course, consider H as forming anions.
NAMING!!!
So finally, now that you know which way these atoms swing in ion formation, we must name them.
Cations are simple. You just give the name of the element and add the word "cation" at the end.
Examples:
K+ is "Potassium cation" and Mg2+is "Magnesium cation"
Anions are a little different. Part of the end of the element's name is removed and an -ide ending is added. The word anion is NOT used.
Examples:
F- is "Fluoride" and N3- is "Nitride"
Below is a picture of a table from the course text (Davis, R. E., & Holt, R. (1999). Modern chemistry: [Gr. 9-12]. Austin, Tex: Holt, Rinehart and Winston.) on page 205. This table shows the common monatomic ions.
You will notice that in the d-block list of atoms, there are some names which include roman numerals, related to the stock system of naming elements and chemical ions. Here is a quick link to the rules (which we will discuss with section 7-2). In summary, the roman numerals are used when there are different possible charges for the cation. Meaning that sometimes, the atom might lose 2 elections (gaining a 2+ charge), while others it could lose 3 (gaining a plus 3 charge). In the first case, a numeral of II would represent the oxidation number of 2, while in the second case, there would be a numeral of III to represent that the ion for that particular element used in this case is the one which has lost 3 electrons.
When the table mentions "d-block," they are referring to the orbitals discussed in chapter 4. the d-block elements are transition metals, and are ofter very unique. Below is the periodic table with the orbitals provided to refresh your memory.
Subscribe to:
Comments (Atom)